Substantia Nigra
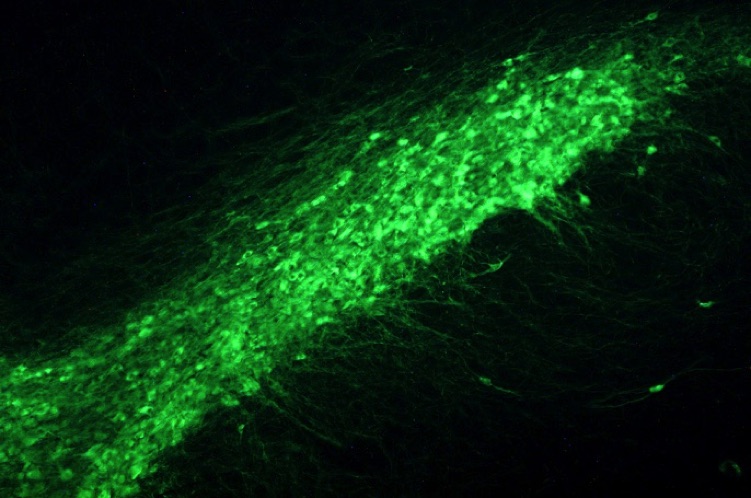
The rat substantia nigra pars compacta showing the dopaminergic neurons and processes revealed by tyrosine hydroxylase immunoctochemistry.
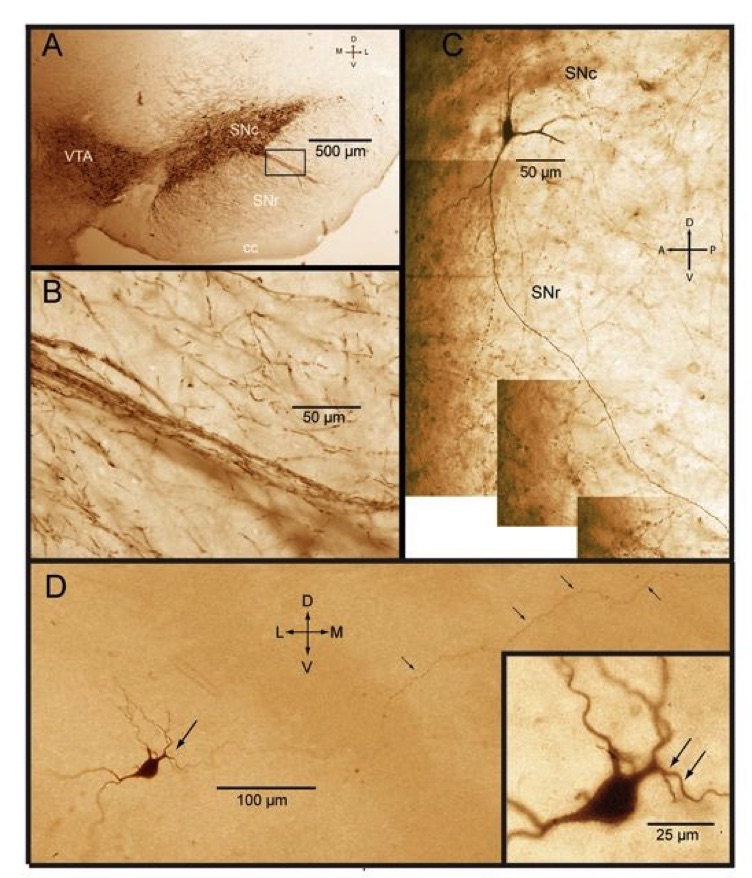
A. TH immunocytochemistry showing dopaminergic (DA) neurons in the substantia nigra pars compacta (SNc) and ventral tegmental area (VTA) immunostained for tyrosine hydroxylase. B. Inset from A showing dendritic fiber fascicles from DA neurons penetrating into pars reticulata (SNr). C. Montage of photomicrgraphs of a biocytin labeled nigral DA neuron showing the typical long ventral dendrite penetrating deep into SNr with little or no branching. D. Nigral DA neuron intracelularly stained with biocytin following electrophysiological identification and recording from a rat brain slice. Tepper and Iribe, unpublished.
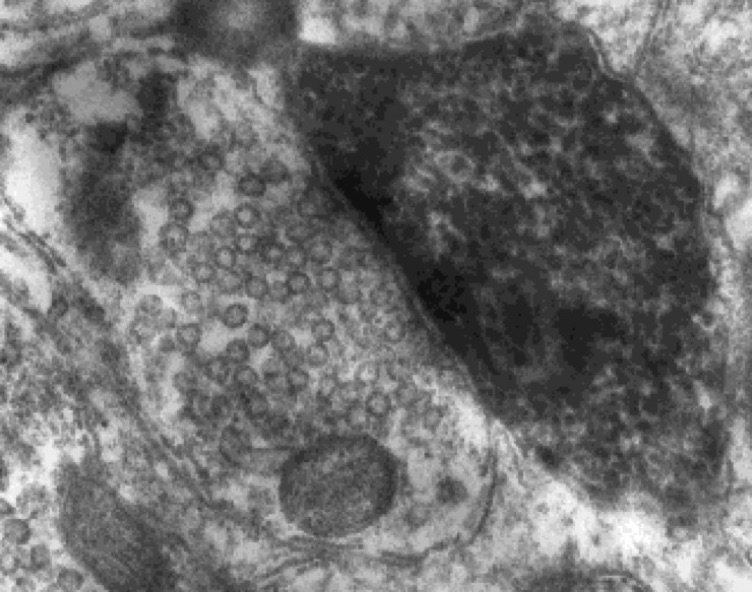
Electron micrograph of a symmetric small round vesicle-containing synapse made onto a dendrite approximately 125 microns from the soma of a substantia nigra dopaminergic neuron intracellularly stained in vivo with HRP. Tepper, unpublished.
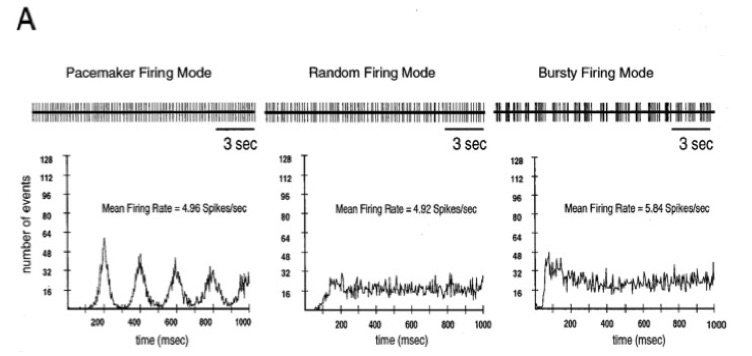
Dopaminergic neurons in vivo fire in three distinct patterns that are easily differentiated in autocorrelation histograms. The three patters actually exist along continuum, with the regular, pacemaker-like at one end, the random or irregular pattern in the middle, and the bursty firing pattern at the other extreme. In anesthetized animals, the random pattern is the most common, followed by the pacemaker pattern, and the burst pattern least common. From Tepper et al., 1995 J. Neurosci. 15:3092-3103.
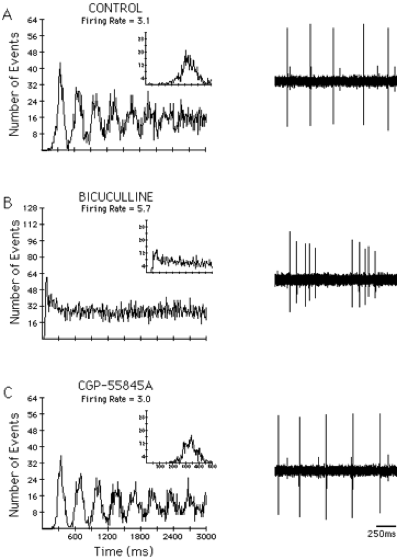
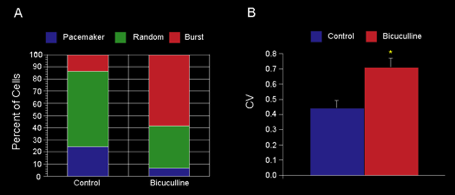
Blocking GABAA inputs to DA neurons makes them fire in the bursty mode whereas blocking GABAB inputs does not. A) Before application of any drug the neuron fired in a pacemaker pattern. B) After application of the GABAA receptor antagonist, bicuculline, the autocorrelogram shifted to an initial peak with a decay to a steady state level indicating a bursty firing pattern. C) Application of the GABAB antagonist CGP-55845A increased the regularity of firing. From Paladini and Tepper (1999) GABAA and GABAB antagonists differentially affect the firing pattern of substantia nigra dopaminergic neurons in vivo. Synapse 32:165-176.
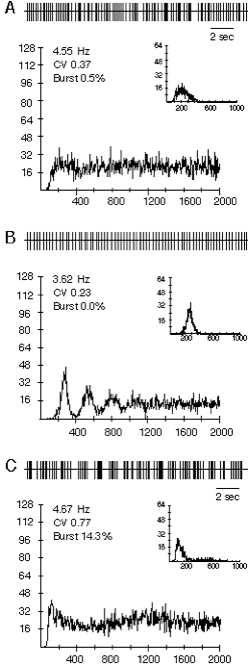
Identical effects on firing pattern can be elicited by manipulating GABAergic inputs to DA cells arising from GP. A. Typical DA neuron showing random firing under control conditions. B. After muscimol-induced inhibition of globus pallidus the firing rate decreased and the firing pattern became pacemaker-like as can be seen in the spike train (upper panels) and the autocorrelograms. C. Infusion of bicuculline into the globus pallidus increased the firing rate of the neuron and not only reversed the pacemaker effect of the prior muscimol infusion but shifted the dopaminergic neuron into the burst firing mode, characterized by the single initial peak in the autocorrelogram, and increased proportion of spikes fired within bursts. These effects are mediated by a disynaptic loop through the axon collateral of the SN pars retculata projection neurons. From Celada, Paladini and Tepper (1999) GABAergic control of rat substantia nigra dopaminergic neurons: Role of globus pallidus and substantia nigra pars reticulata. Neuroscience 89:813-825.
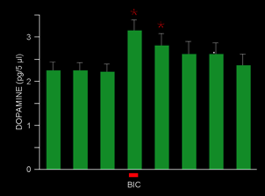
In a subsequent experiment the burst firing in DA neurons caused by pallidal excitation was replicated, this time with a microdialysis probe in the striatum. The burst firing, which was accompanied by only a relatively small 14% average increase in firing rate, produced a very large increase in basal DA levels in striatum, thus showing that changes in firing pattern elicited by alterations in pallidal output similar to those to occurring spontaneously in freely moving animals cause powerful modulation of dopamine release by disinhibiting the pars reticulata input to the dopamine neurons. Lee, Abercrombie, and Tepper (2004) Pallidal control of nigral dopaminergic neuron firing pattern and its relation to extracellular neostriatal dopamine. Neuroscience 129:481-489.
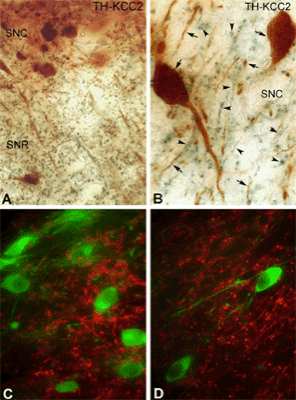
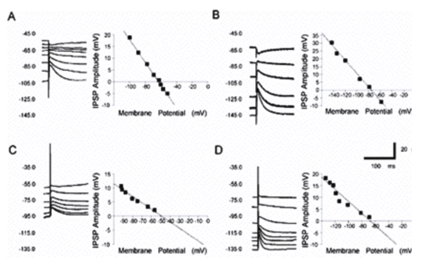
GABAA mediated responses are different in nigral DAergic and pars reticulata neurons. The DAergic neurons (TH+) lack the potassium-chloride cotransporter (KCC2) which is responsible for maintaining the hyperplarizing chloride gradient in most mature neurons. (LEFT) Light micrographs demonstrate the localization of KCC2 in non-DAergic cells of the rat SN. A-B) Segregation of TH-immunolabeled neurons (brown) and KCC2-immunopositive dendrites (blue-black) in both the SNc and SNr. Both somata and dendrites of the DAergic neurons are labeled for TH (arrows). KCC-2 was found only in the dendrites of non-DAergic cells (arrowheads). C-D) Double-immunofluorescent staining shows a mutually exclusive distribution pattern for TH and KCC2. None of the TH-positive (green) dendrites or somata are outlined by KCC2 immunoreactivity (red). Scales: 50 µm (A,C,D), 25 µm (B). (RIGHT) Pharmacologically isolated GABAA receptor-mediated IPSPs in response to stimulation of the SNr in vitro. A) Representative current clamp recordings of a DAergic neuron at various membrane potentials show an IPSP in response to stimulation of the SNr. Inset is a plot of the IPSP amplitude versus the membrane potential. The plot of the regression line revealed that the reversal potential of the IPSP was 62.9 mV. B) Representative current clamp recordings of IPSP in GABAergic SNr neuron shows significantly more hyperpolarized reversal potential of75.8 mV. C, D) In bicarbonate ion free slice buffer, the GABAA receptor mediated IPSP reversal potential in a representative DAergic neuron (C) becomes significantly less hyperpolarized at -50.2 mV, whereas there is no significant change in a non-DAergic neuron in which the IPSP reverses at -70.6 mV (D) indicating that the slightly hyperpolarizing Cl- gradient in DAergic neurns comes from the sodium-dependent anion exchanger (NDAE). Modified from Gulácsi, Lee, Sík, Viitanen, Kaila, Tepper and Freund (2003) Cell type-specific differences in chloride-regulatory mechanisms and GABAA receptor mediated inhibition in rat substantia nigra. J. Neurosci. 23:8237-8246.
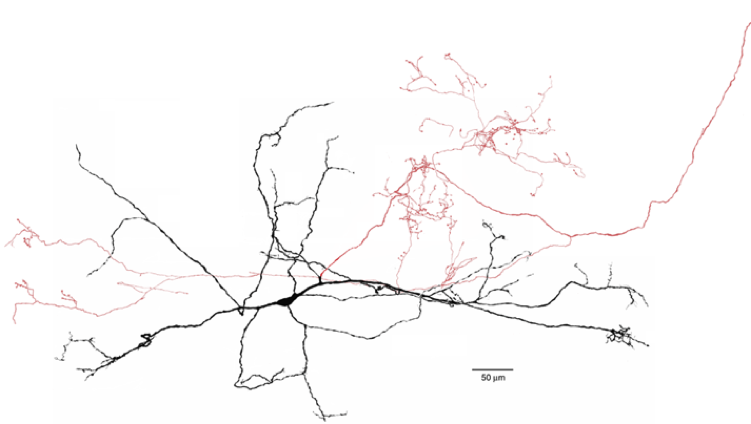
Substantia nigra pars reticulata GABAergic projection neuron juxtacellularly stained in vivo with biocytin. Note the overall sparseness of the local axon collaterals that form a few very dense arborizations, separated by long stretches of non-varicose axons. Shah and Tepper, unpublished.
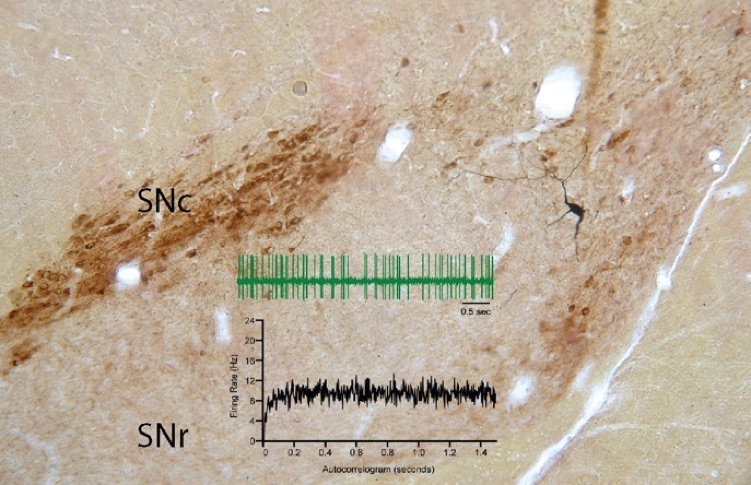
SNr GABAergic projection neuron juxtacellularly stained in vivo with biocytin. Section is counterstained for TH showing the location of the DA neurons in SNc. Insets show a sample of the spontaneous firing of this cell and an autocorrelogram of the spontaneous activity exhibiting the irregular, non-bursty firing pattern typical of SNr projection neuron in anesthetized rats. Shah and Tepper, unpublished.
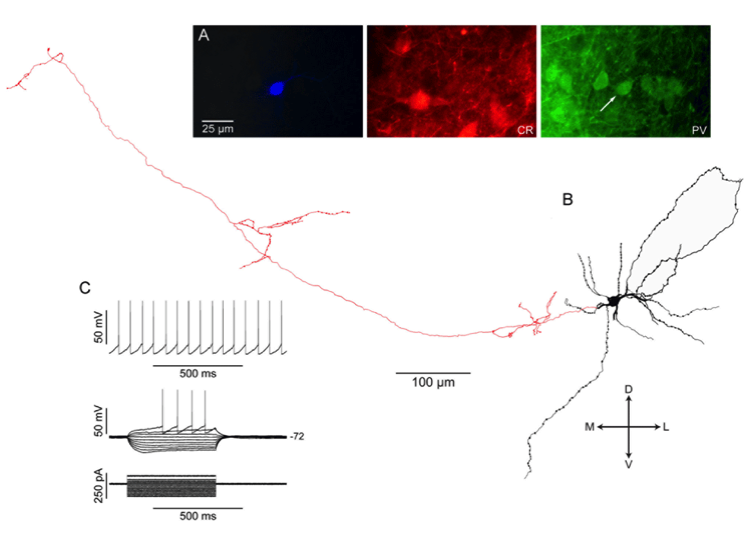
SNr GABAergic projection neuron from rat slice stained with biocytin after whole cell recording. Fluorescent photomicrographs show that this neuron co-localizes parvalbumin but not calretinin, like about 50% of SNr neurons. The other half colocalize CR but not PV. Only a tiny fraction co-localize both calcium binding proteins. All SNr projection neuron exhibit intrinsic electrophysiological properties similar to those shown above. From Lee and Tepper (2007) Morphological and physiological properties of parvalbumin- and calretinin-containing gamma-aminobutyric acidergic neurons in the substantia nigra. J. Comp. Neurol. 500:958-972.